Bases. Jars containing calcium carbonate (Ca2CO3), copper oxide (CuO) and sodium hydroxide (NaOH). These compounds are classified as bases, because th Stock Photo - Alamy
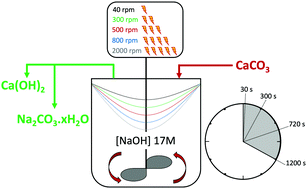
![PDF] Regeneration of Sodium Hydroxide from a Biogas Upgrading Unit through the Synthesis of Precipitated Calcium Carbonate: An Experimental Influence Study of Reaction Parameters | Semantic Scholar PDF] Regeneration of Sodium Hydroxide from a Biogas Upgrading Unit through the Synthesis of Precipitated Calcium Carbonate: An Experimental Influence Study of Reaction Parameters | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/00e03ec2cffecd61e840107d0d9fcb645c1e1c00/3-Figure1-1.png)
PDF] Regeneration of Sodium Hydroxide from a Biogas Upgrading Unit through the Synthesis of Precipitated Calcium Carbonate: An Experimental Influence Study of Reaction Parameters | Semantic Scholar
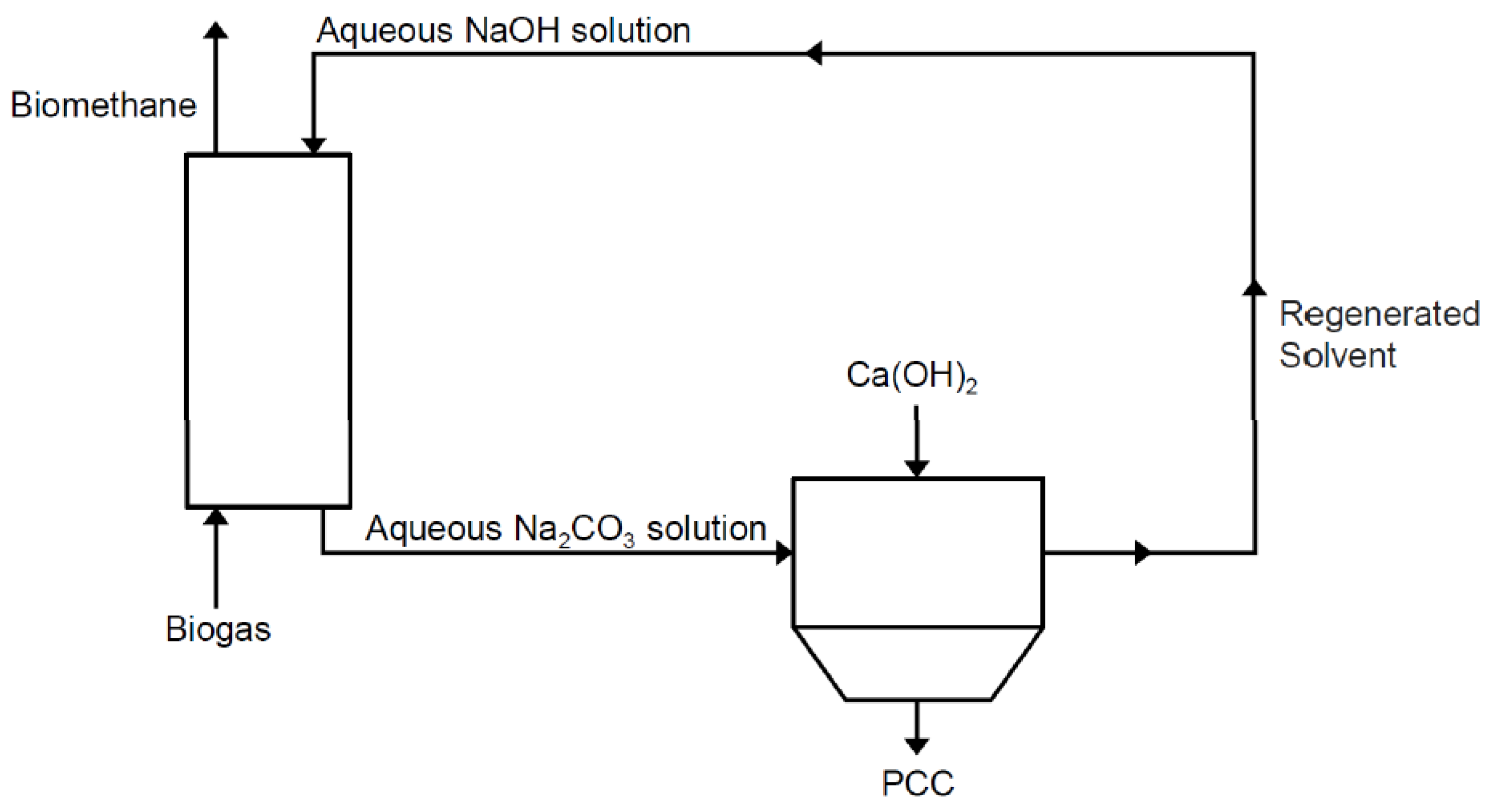
Processes | Free Full-Text | Regeneration of Sodium Hydroxide from a Biogas Upgrading Unit through the Synthesis of Precipitated Calcium Carbonate: An Experimental Influence Study of Reaction Parameters
Decarbonisation of calcium carbonate at atmospheric temperatures and pressures, with simultaneous CO2 capture, through productio

Write the balanced chemical equations for the following reactions.A Calcium hydroxide + Carbon dioxide → Calcium carbonate + waterB Zinc + Silver nitrate → Zinc nitrate + SilverC Aluminium + copper chloride
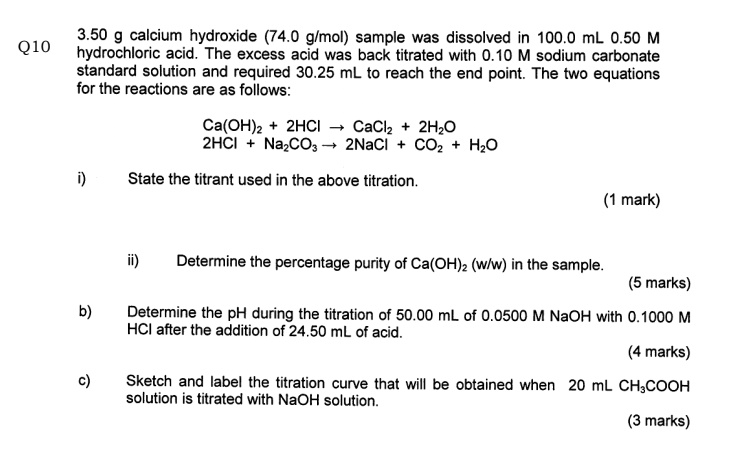
SOLVED: 3.50 g calcium hydroxide (74.0 glmol) sample was dissolved 100.0 mL 0.50 M hydrochloric acid, The excess acid was back titrated with 0.10 M sodium carbonate standard solution and required 30.25
PDF) Comparison of the efficiencies of sulfur dioxide absorption using calcium carbonate slurry and sodium hydroxide solution in an ALT reactor
Decarbonisation of calcium carbonate at atmospheric temperatures and pressures, with simultaneous CO2 capture, through productio

For the reaction between sodium carbonate and calcium hydroxide to form a precipitate. Write a net - Brainly.in

a) Substitute formulae for names and balance the following equation: Calcium carbonate reacts - YouTube

SEM of CaCO 3 particles obtained by mixing calcium hydroxide solution... | Download Scientific Diagram
A solution of Y was slowly added to a solution of X. The graph shows how the pH of the resulting solution changed. (a) (i)

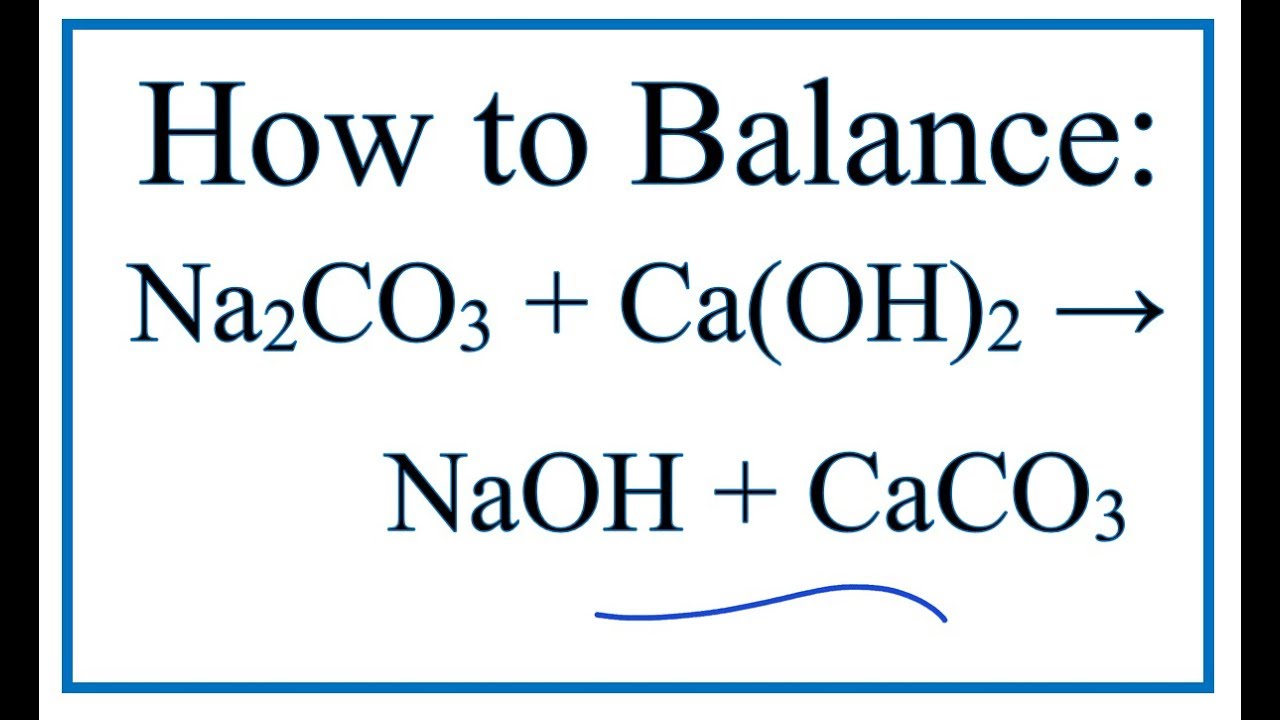
SOLVED: If 3.5 moles of the sodium carbonate react with excess calcium carbonate, how many grams of sodium hydroxide will be produced? Na2co3 + Ca(OH)2 NaOH + Caco3

Reaction of calcium carbonate minerals in sodium silicate solution and its role in alkali-activated systems - ScienceDirect
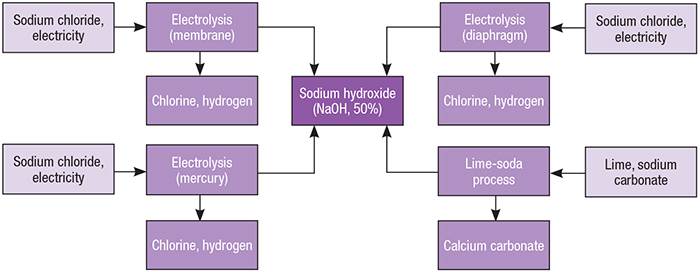
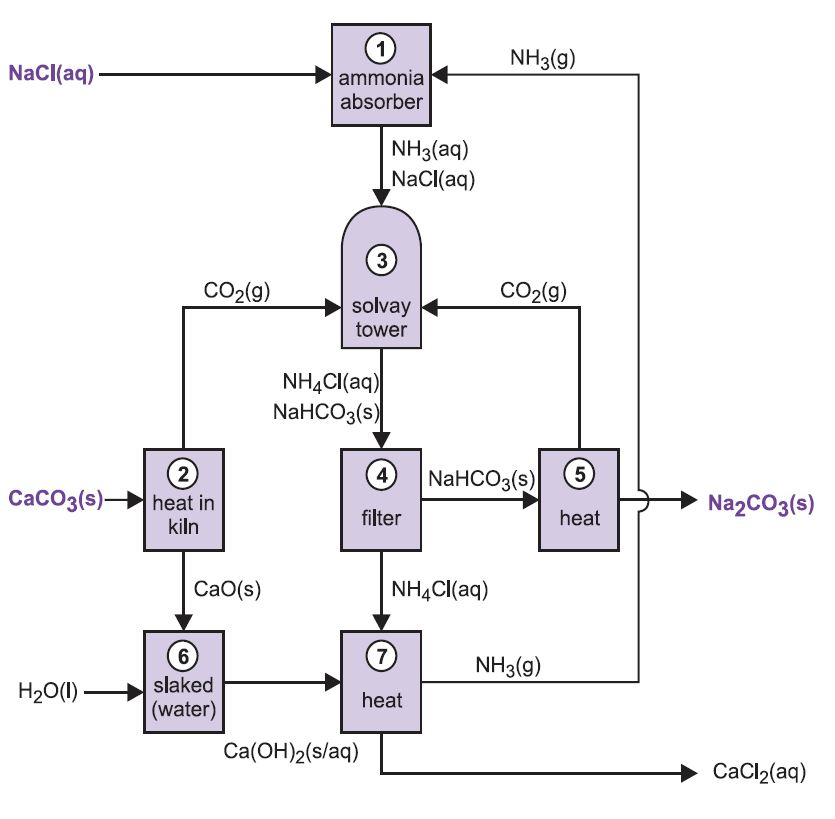
![PDF] Recovery of Sodium Hydroxide from Trona Ore and Calcium Carbonate as Raw Materials | Semantic Scholar PDF] Recovery of Sodium Hydroxide from Trona Ore and Calcium Carbonate as Raw Materials | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/c111a7b476ee6999fdd843657756ea6a4f670b83/3-Figure1-1.png)
PDF] Recovery of Sodium Hydroxide from Trona Ore and Calcium Carbonate as Raw Materials | Semantic Scholar


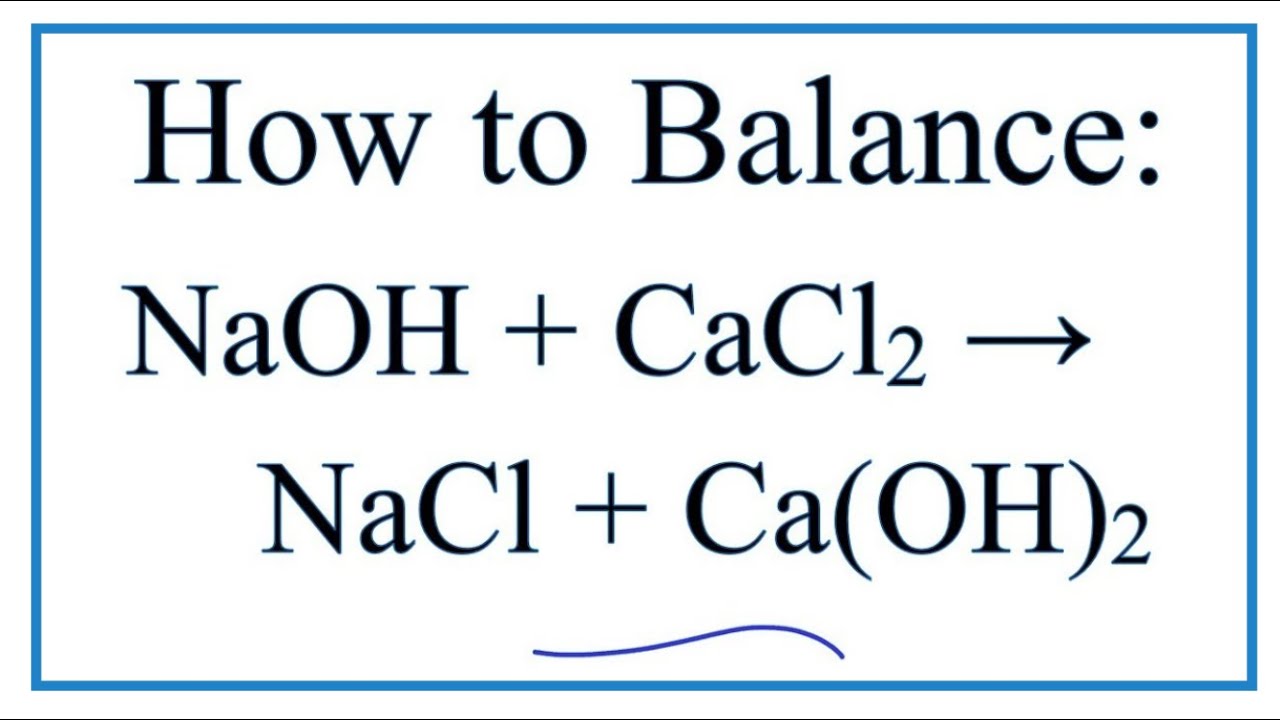

![ANSWERED] Calcium hydroxide reacts with aqueous sod... - Physical Chemistry ANSWERED] Calcium hydroxide reacts with aqueous sod... - Physical Chemistry](https://media.kunduz.com/media/sug-question/raw/51753485-1659180238.598407.jpeg)