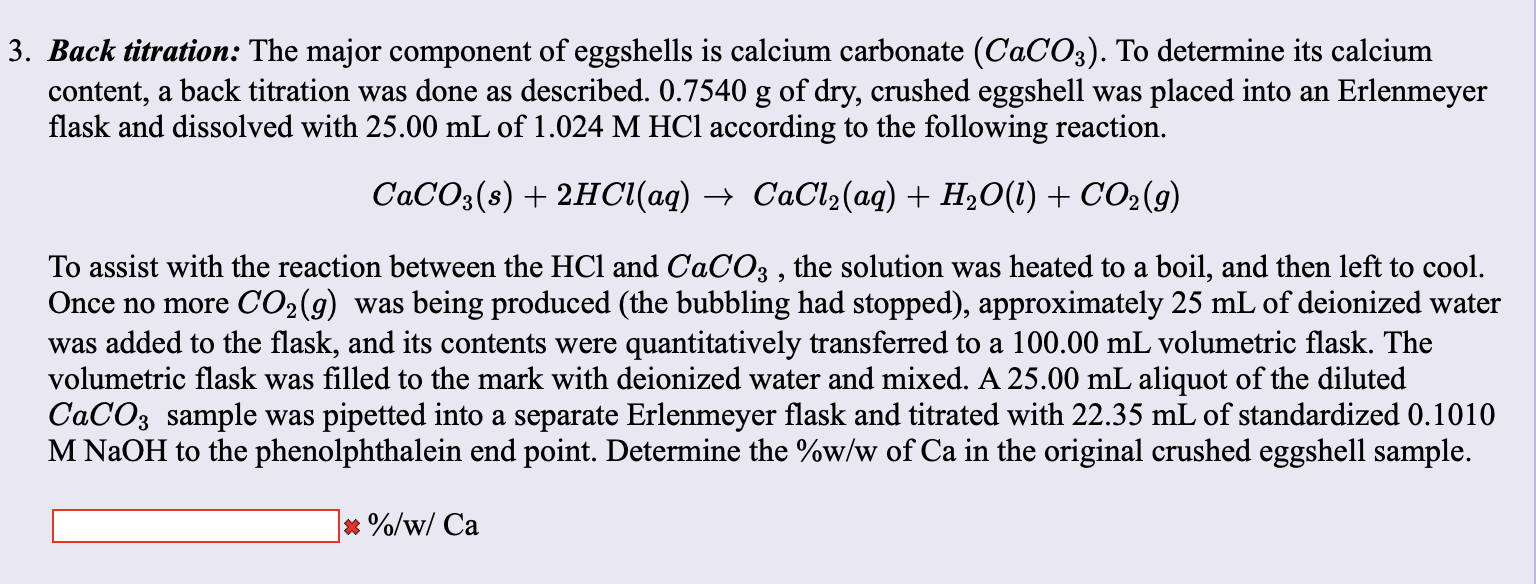
Titration curves of calcium carbonate precipitation in the presence of... | Download Scientific Diagram

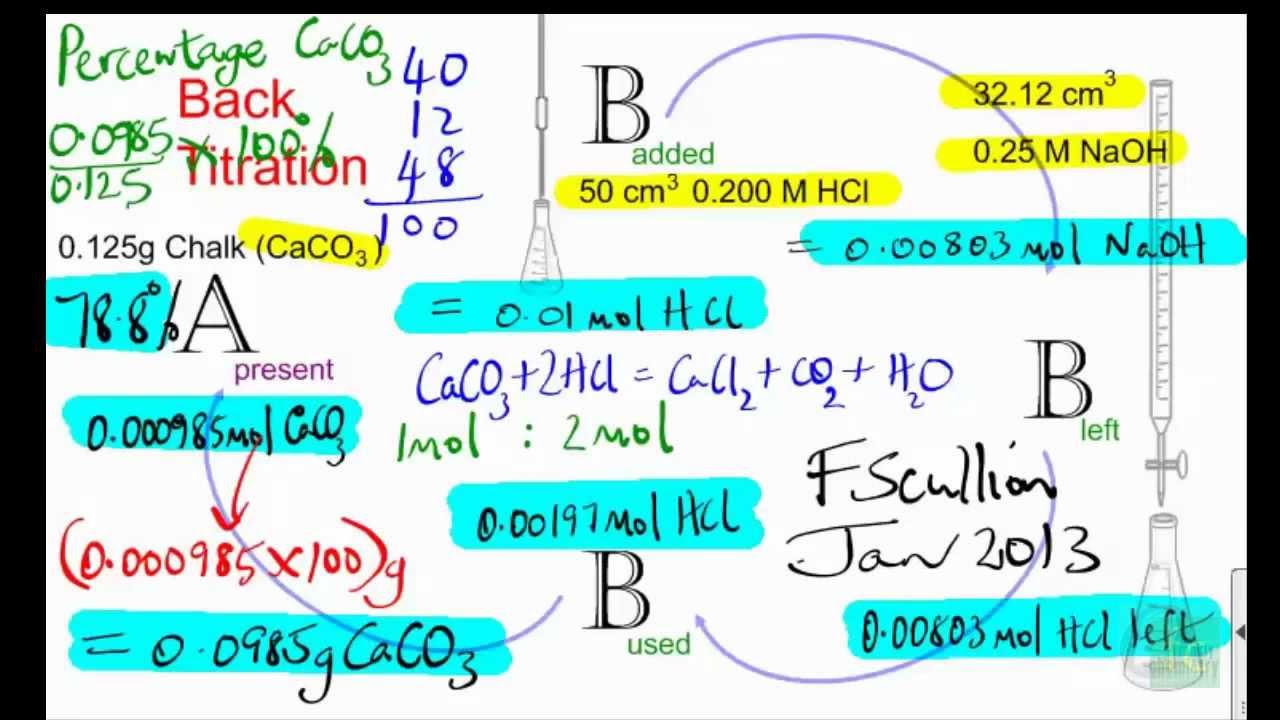
Determination of Amount of CaCO3 in Eggshell by Back Titration Method | PDF | Hydrochloric Acid | Titration

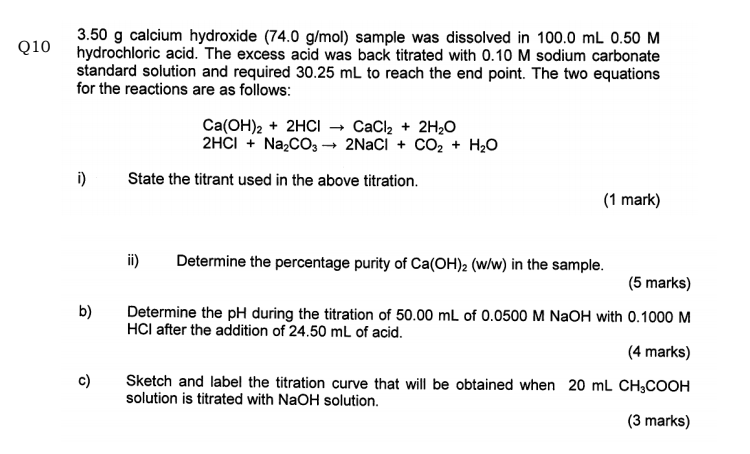
DOC) OBJECTIVE To determine the calcium carbonate content in eggshell | Sharifah Hana - Academia.edu

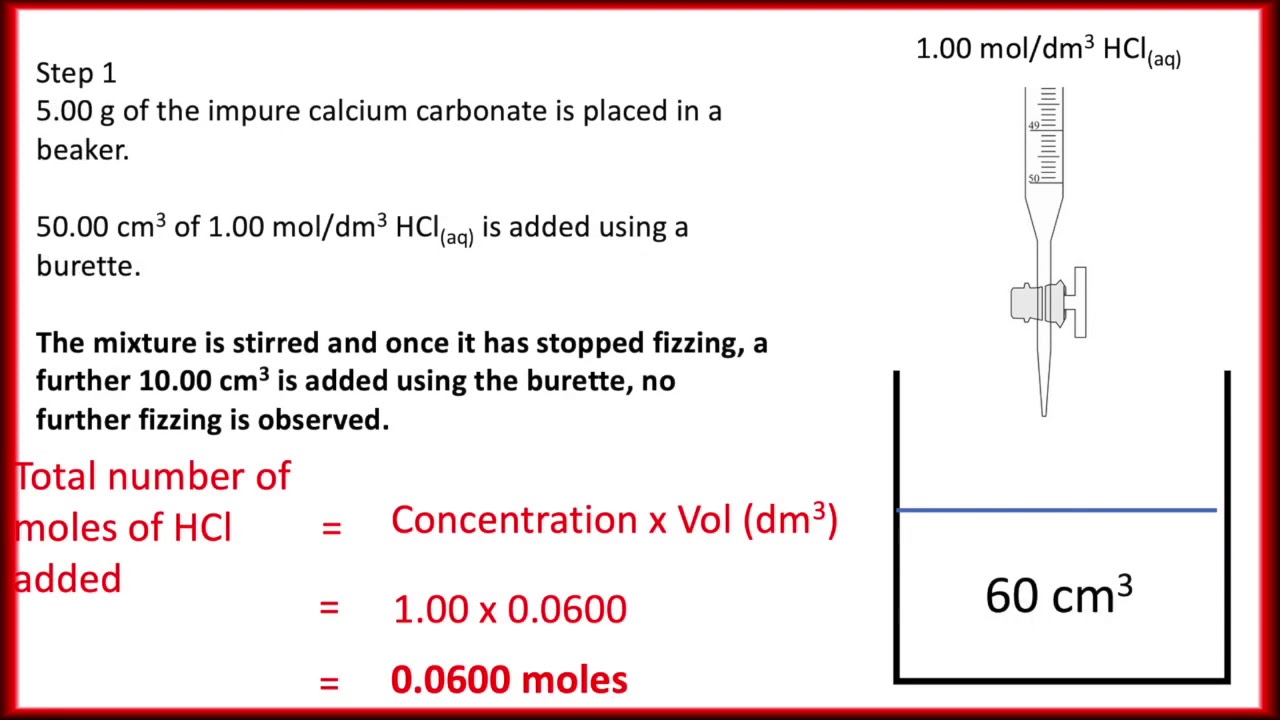
Experiment Report - back titration.docx - Experiment Report Finding the Purity of CaCO3 using Back Titration Introduction In order to calculate the | Course Hero






![Lab: Back titration to determine the % CaCO3 in eggshell DATA COLLECTION [IB CHEMISTRY] - YouTube Lab: Back titration to determine the % CaCO3 in eggshell DATA COLLECTION [IB CHEMISTRY] - YouTube](https://i.ytimg.com/vi/fH-RzzDN0Lg/hq720.jpg?sqp=-oaymwEhCK4FEIIDSFryq4qpAxMIARUAAAAAGAElAADIQj0AgKJD&rs=AOn4CLAUKQdGwq7plYa7h8dYUpGwcEOhrA)