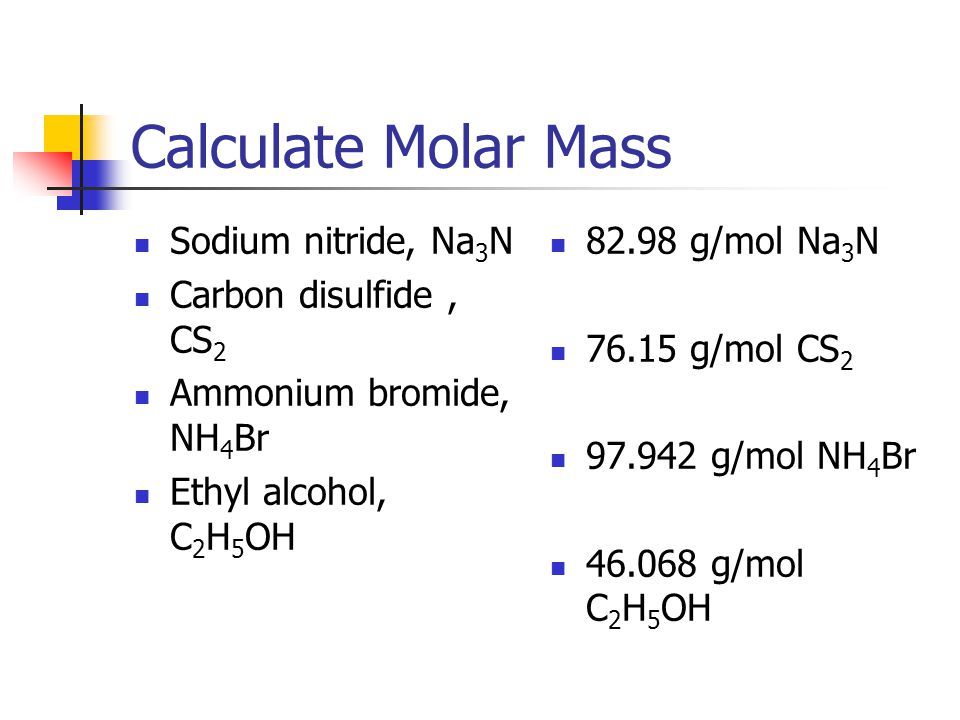
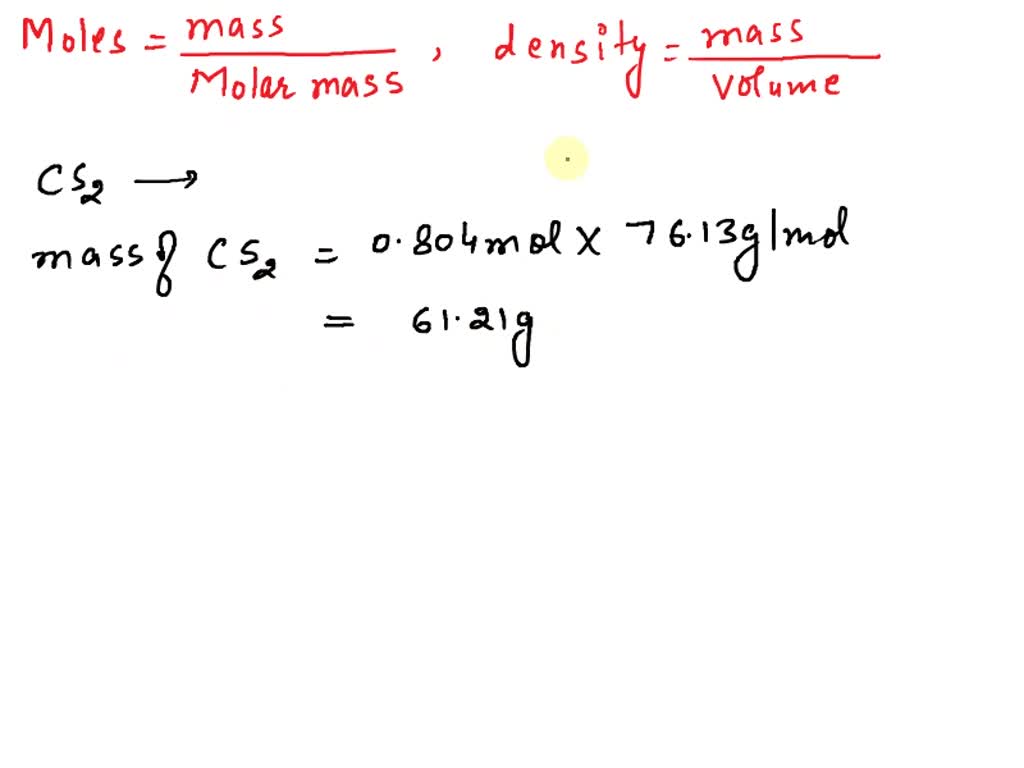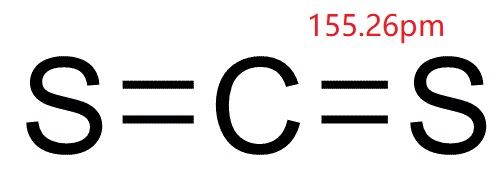SOLVED: QUESTION 8 Determine the change in boiling point for 373.9 g of carbon disulfide (Kb = 2.349C kg/mol) if 35.0 g ofa nonvolatile, nonionizing compound is dissolved in it The molar

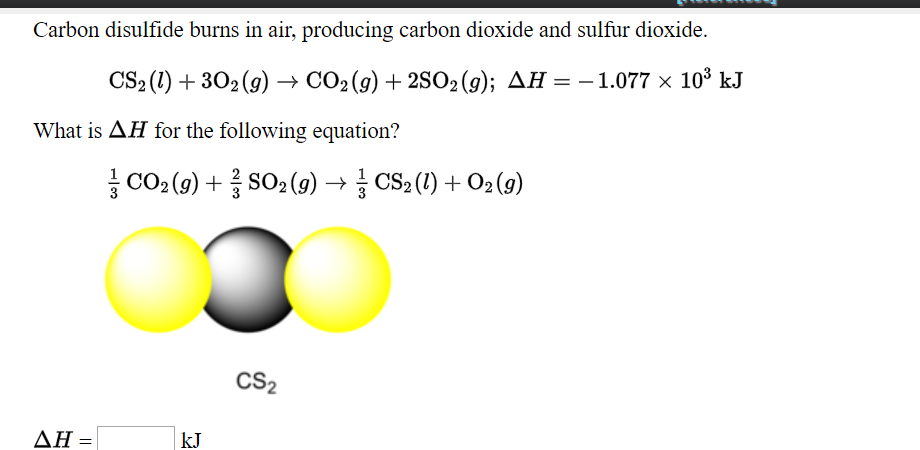
Given the following data, estimate the boiling point of carbon disulfide, CS2 , assuming that ?So and ?Ho are temperature-independent. Table showing the endothermic heat and the Standard entropy for | Homework.Study.com

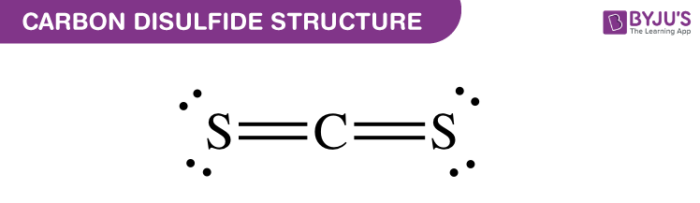
What is carbon disulfide?. Carbon disulfide is also known as… | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium