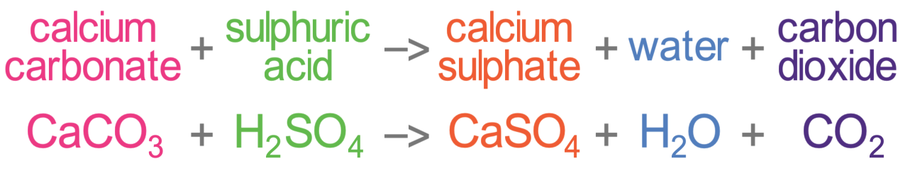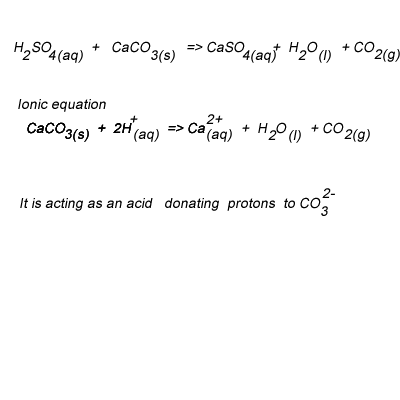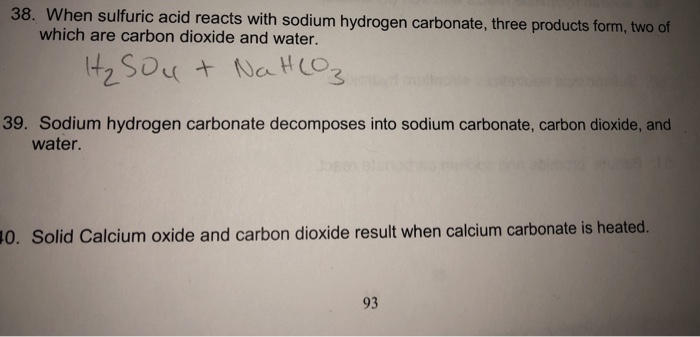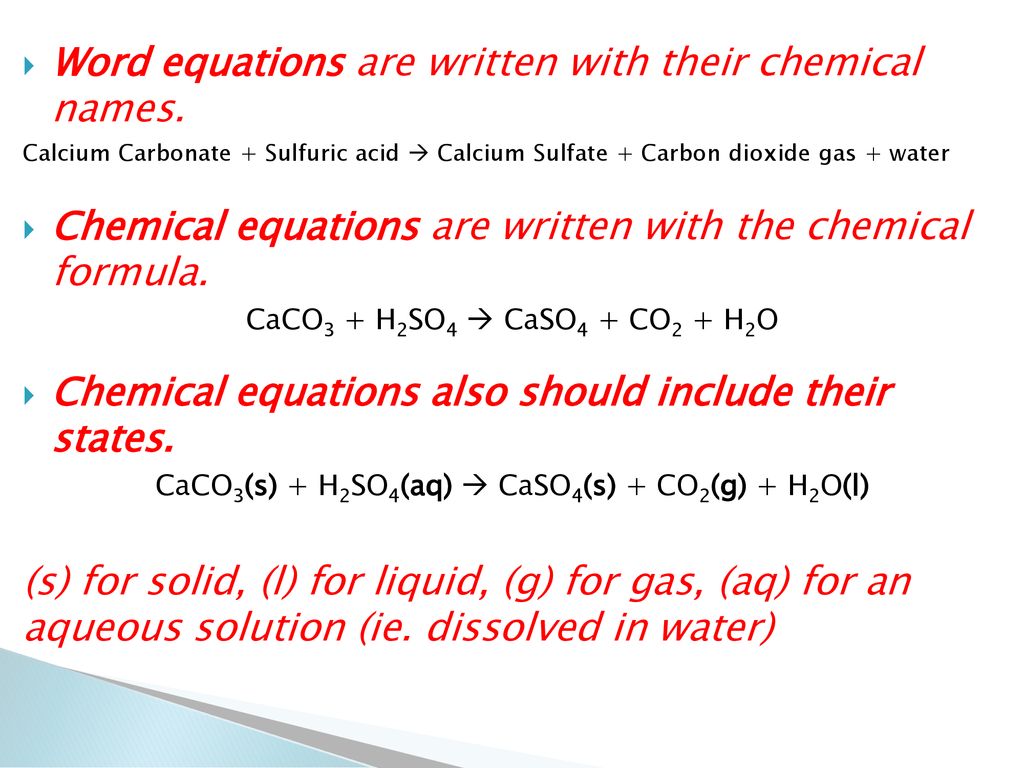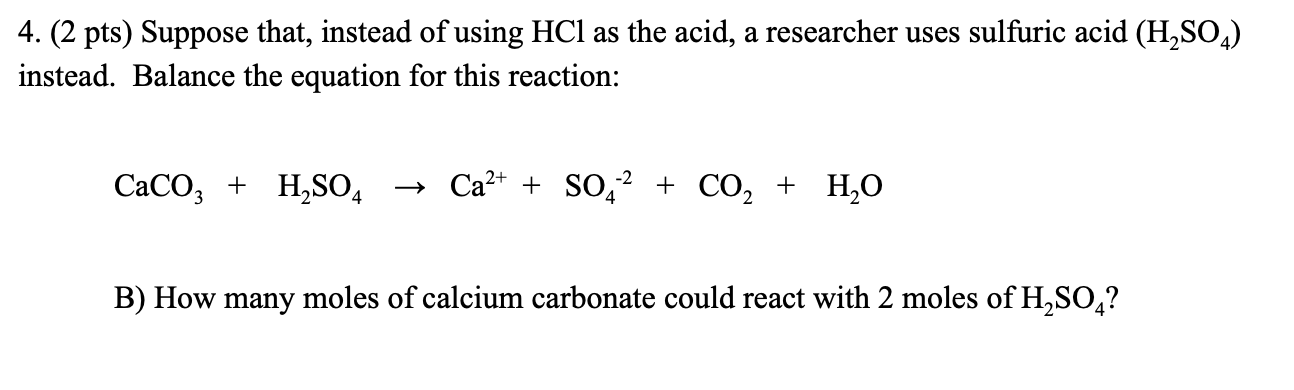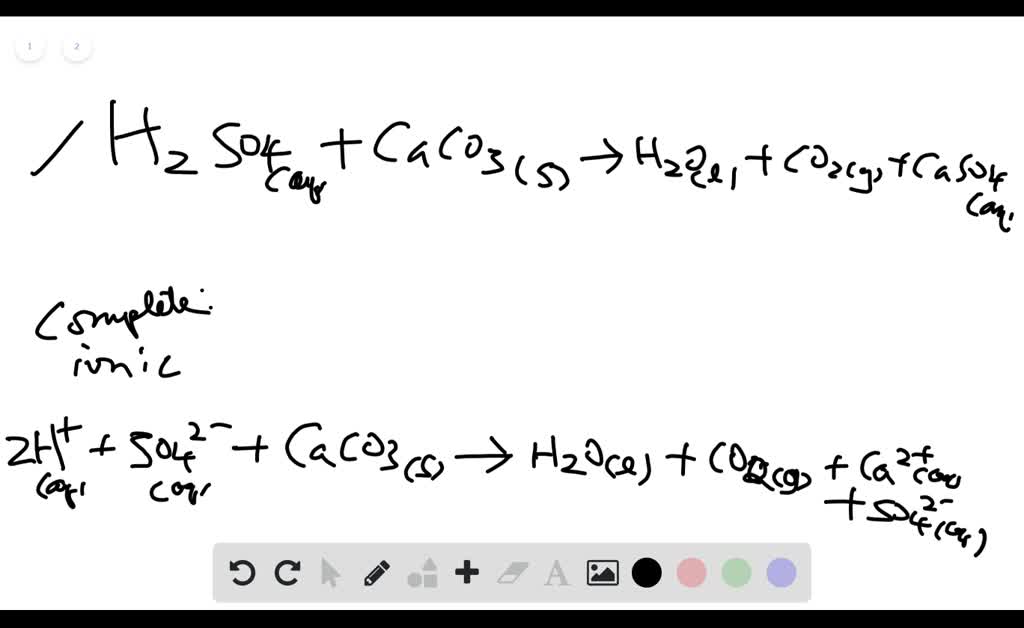
SOLVED:Write complete ionic and net ionic equations for the reaction between sulfuric acid (H2 SO4) and calcium carbonate (Ca CO3) . H2 SO4(aq)+ CaCO3(s) →H2 O(l)+CO2(g)+CaSO4(aq)

Question Video: Writing a Net Ionic Equation for the Reaction of Solid Calcium Carbonate with a Hydrochloric Acid Solution | Nagwa